Research in the Wang Lab
Defining novel therapeutic strategies for cardiovascular diseases, the leading cause of death worldwide, has long been a key area in my own work. By integrating the state-of-art technologies including super-resolution microscopy, single-molecule pulldown (SiM-pull), fluorescence resonance energy transfer (FRET) Microscopy (FRET), diverse animal models (myocardial infarction) induced ischemic heart model, high-fat-diet-induced diabetic cardiomyopathy model ,and chronic β-adrenergic stimulation induced heart failure model), and transgenic mice, I have provided multiple promising molecular targets for rescuing cardiac dysfunction targeting local adrenergic signaling at nanodomains and contributed to the successful translation of a drug candidate, SCM-198 from bench to bedside.
This page lists some of the past projects and on-going future projects. Please feel free to contact wangy6 AT sustech.edu.cn for discussion.
Research Topic 1
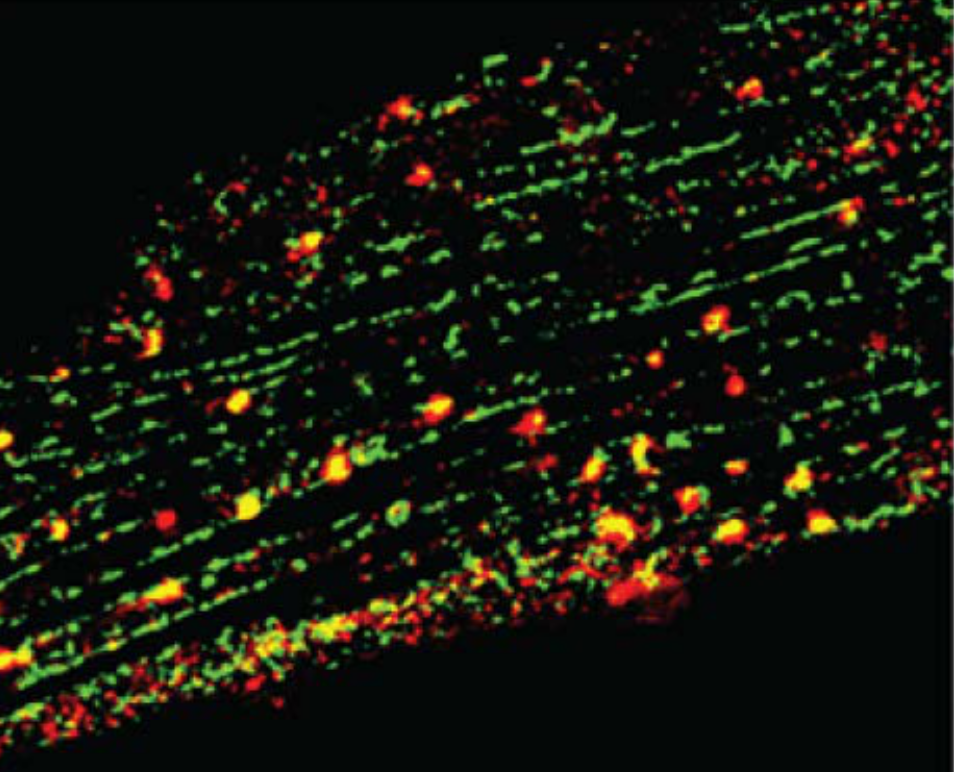
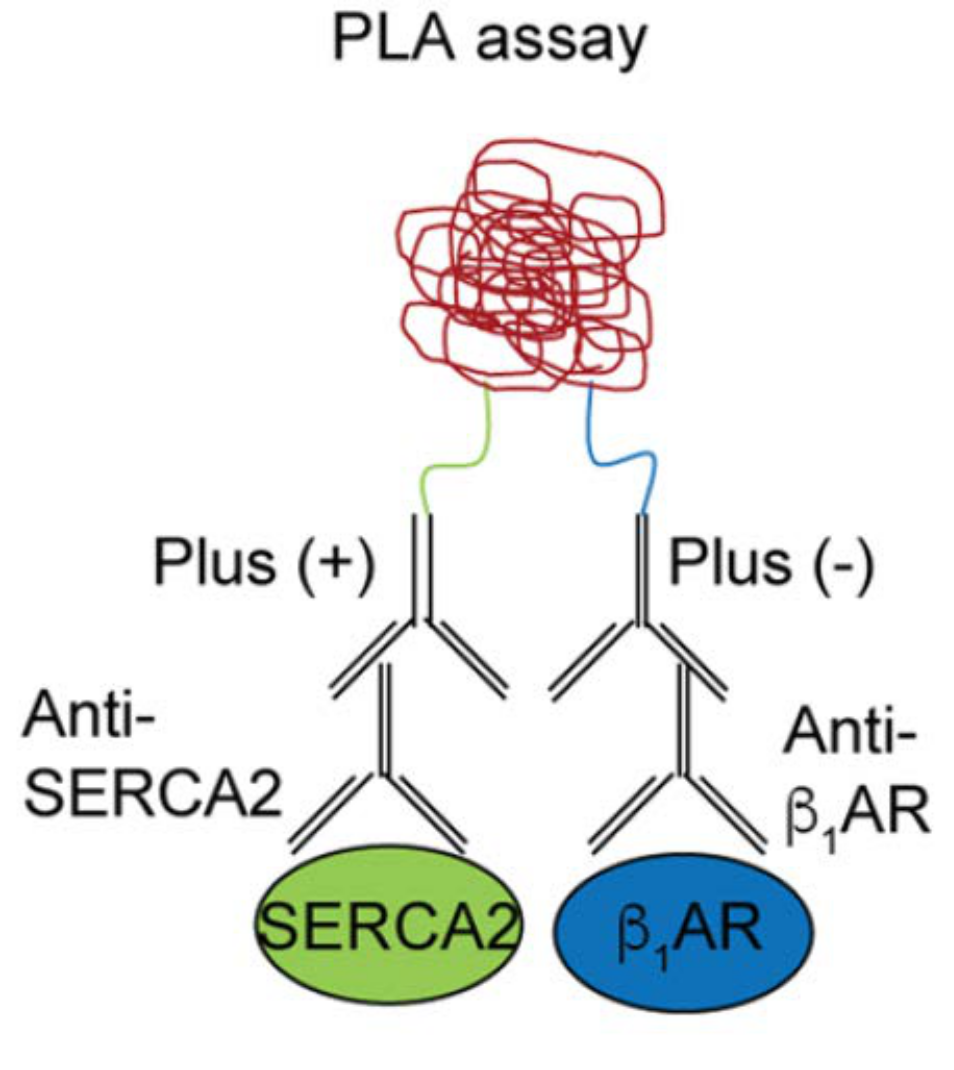
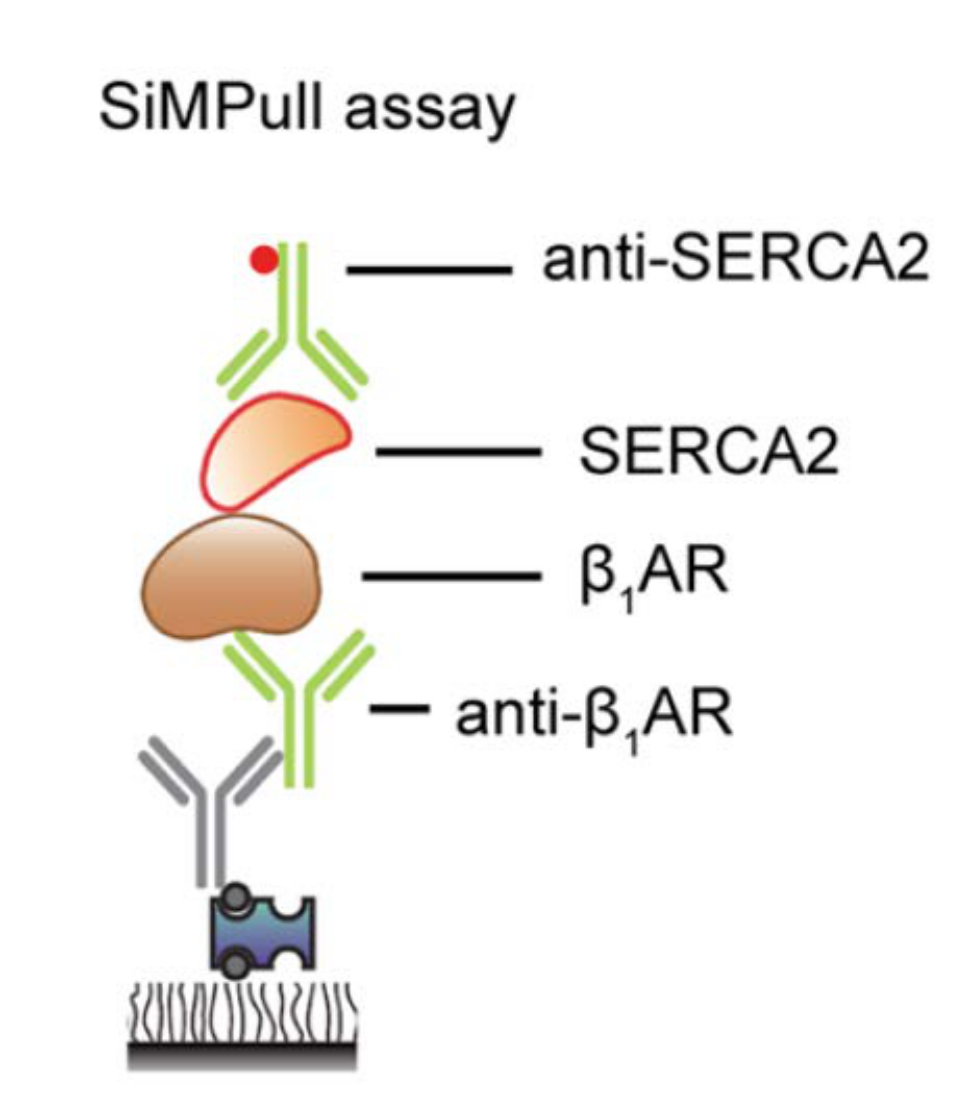
Cardiac contraction is orchestrated by compartmentalization of β-adrenergic signaling at distinct subcellular nanodomains. The highly organized architecture in cardiomyocytes provides spatial context for the subcellular localized cAMP/PKA signaling in the regulation of sophisticated EC-coupling, a process of heart to contract and relax. To visualize the cAMP/PKA nano-domains, we have developed a serial of genetical encoded Förster resonance energy transfer (FRET)-based biosensors anchoring at the distinct subcellular locations including plasma membrane (PM), sarcoplasmic reticulum (SR), myofilament (MF), and nuclear envelop. By collaborating with the world-leading experts, Prof. Donald M. Bers and Prof. Johannes W Hell and utilizing these powerful tools as well as the cutting-edge technologies, super-resolution imaging, proximity ligation assay (PLA, Figure 2), and singlemolecule pulldown (SIM), I discovered a pool of intracellular β-adrenergic receptors (βAR) at the SR is essential for PKA-phosphorylation of phospholamban and cardiac contractility (Wang et al 2021a Circulation Research).
Research Topic 2
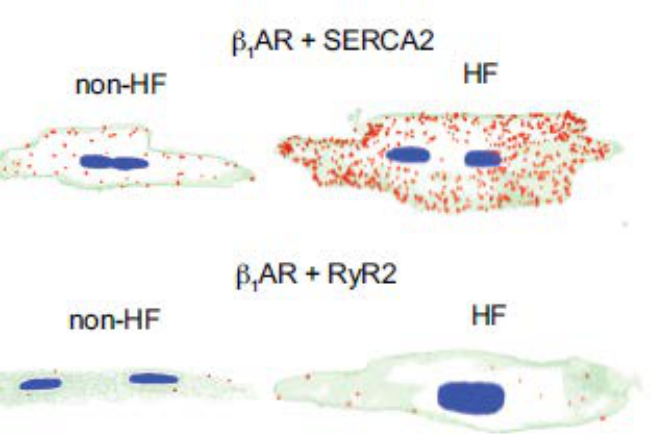
Desensitization of β1AR signaling and depressed cardiac contractility are hallmarks of heart failure (HF). Therefore, we asked whether the SR-localized β1ARs are engaged in the HF. Using the HFrEF (HF with reduced ejection fraction) model induced by chronic adrenergic stimulation and an ischemic HF model induced by myocardial infarction, I found the elevation of monoamine oxidase A (MAO-A) desensitized intracellular β1ARs signaling in HF (Wang et al 2021b Circulation Research). Knockout or inhibition of MAO-A resensitizes intracellular β1AR signaling and inotropic responsiveness in failing mice (Wang, Circulation, Under review). My investigation of MAO-A-mediated intracellular β1AR/PKA signal cascade was granted by the prestigious American Heart Association Postdoctoral Fellowship.
Research Topic 3
The integrative control of intracellular catecholamine for subcellular β1AR signaling and cardiac function is lacking of investigation and presents as a promosing therapeutic target for HF. I have determined the regulation of compartmentalized β1AR-PKA signaling at the SR and plasma membrane (PM) microdomains by organic cation transporter 3 (OCT3) and monoamine oxidase A (MAO-A), two critical modulators of catecholamine uptake and homeostasis.
Our results show that MAO-A and OCT3 act in concert to fine-tune the intracellular β1AR-PKA signaling and cardiac fight-or-flight response. Further, we reveal a drug contraindication between anti-inflammatory corticosterone and anti-depressant MAOi in modulating adrenergic regulation in the heart, providing novel perspectives of these drugs with cardiac implications.
Research Topic 4